
BETTER Phase IIB study will examine the effects of adaptive computer brain training on cognition in older subjects.
Despite a growing number of studies demonstrating cognitive benefits from cognitive training, there has been very limited documentation of the long-term outcomes of computer-delivered cognitive training, measured by behavioral, neurological and life-outcomes aspects of brain health status in rigorously controlled trials. The HBC Lab’s Brain Enhancement Training Towards Elders Resilience to Aging (BETTER Aging - Phase IIB) trial will help build stronger and more accessible prevention strategies by testing the optimal cognitive training program for resistance and resilience to brain aging.
This BETTER Phase IIB trial follows up on our Phase II study which assessed cognitive aging on cognitive tasks sensitive to brain aging. Performance outcomes were tested immediately after training and 6-months after the end of training. Phase IIB now examines the longer-term benefits of a computerized training program’s ability to improve cognitive and functional performance, as well as brain structure and function. The study will also measure the impact of the training on pathologies associated with Alzheimer’s Disease. The study is a collaboration between three research sites, including the coordinating site Posit Science Corporation (PSC), the HBC lab at the University of Iowa, and the Lifespan Neuroscience and Cognition Lab at the University of Texas-Dallas. Like Phase II, Phase IIB is funded exclusively by the National Institutes on Aging.
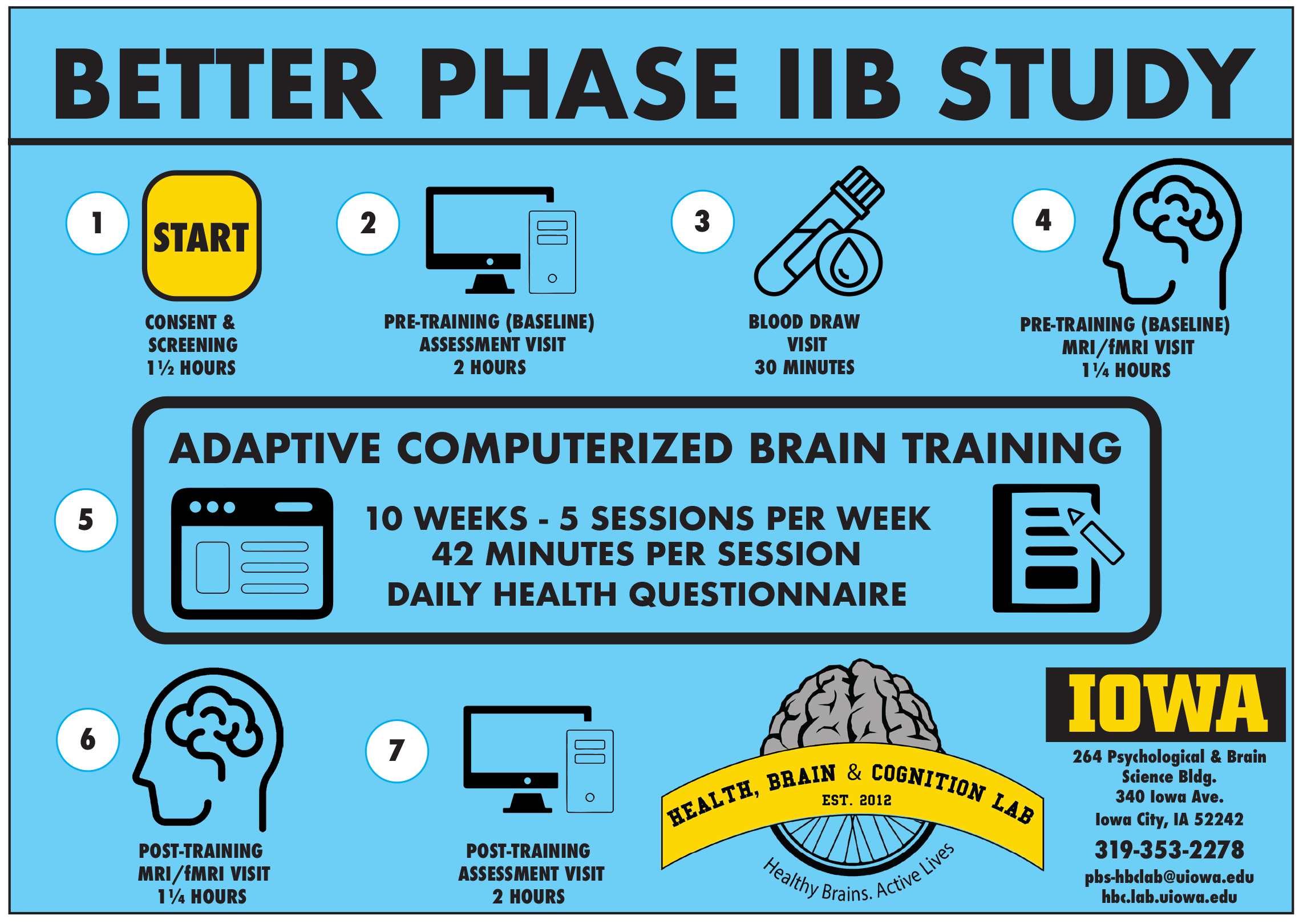
The target population is limited to those 70 years of age or older. The trial is open to persons of all races, ethnicities, and genders. Across both study sites, our goal is to enroll 124 Phase II subjects who completed training five years ago, plus 60 newly-recruited, age-matched participants. Both groups will receive 10 weeks of home-based computerized cognitive training.
Outcomes will provide deeper understanding of the neurobehavioral status of a study population five years after the initial 10 weeks of program completion and of the benefit of the booster training and its interaction with prior-training.
To evaluate prolonged program efficacy five years after completion of earlier training, we will assess participants in three areas:
- Cognitive and functional performance
- Structural (white matter integrity and brain volume) and functional brain changes (resting state MRI and task-fMRI)
- Neurodegeneration assessed with blood-based biomarkers (plasma profile of phosphorylated and total tau proteins, Aβ1-42/Aβ1-40) predictive of AD risk.
We will also assess whether certain patterns of genetic variation affect things like brain structure, cognition, and function in participants.
To determine the efficacy of “booster training” after five years from initial training completion, all Phase II participants and newly recruited participants will be assessed for cognitive performance and brain structure and function before and after 10 weeks of training. All participants will complete intense adaptive training as their “booster” in this Phase IIB study.
We will examine 1) the general booster efficacy across groups by comparing performance of pre- and post-booster training, and 2) the interaction between initial training (adaptive, casual games, untrained) and booster training by comparing the change scores between groups. In an exploratory analysis, we will examine the influence of chronological age on training efficacy by examining whether training benefits of adaptive training differ based on age of training.
At the beginning of each training session, participants report their physical activity, diet, social interaction, sleep quality, and memory lapses.
Participants will be asked to use the computerized program for 42 minutes per session, up to five sessions per week, over 10 weeks. Several elements of flexibility are allowed in the schedule to accommodate the challenges that older adults with age-related cognitive decline may face.
Compensation will occur following completion of each study in-person visit and weekly for the training sessions. Participants who complete all five in-person visits and all 50 training sessions will be compensated $550 maximum for their participation in the study. Participants who withdraw from the study will be compensated for all the training sessions and study visits they have completed.
Some participants may be offered a device and/or wireless card to complete training sessions at their residence depending on availability. Participants who prefer to use their personal device and personal Internet access will be permitted to do so. Each study participant will be remotely supervised by a Site Cognitive Remediation Coach. They will have phone or email contact at least once per week, and may adjust the contact depending on each participant’s needs
The study is pre-registered on clinicaltrials.gov and the status of recruitment will be updated regularly at the study site on ClinicalTrials.gov: https://www.clinicaltrials.gov/study/NCT05599490